Red Light Therapy: Comprehensive Evidence-Based Analysis

The therapeutic use of specific wavelengths of red and near-infrared light has evolved from experimental treatment to evidence-supported therapy across multiple medical conditions. Red light therapy demonstrates moderate-to-strong clinical evidence for hair loss, pain management, and wound healing, with well-established safety profiles and FDA clearance for several applications. However, the field faces significant challenges in standardization and evidence quality that limit broader medical adoption.
This comprehensive analysis reveals a therapy with solid scientific foundations but uneven clinical validation, requiring careful evaluation of evidence quality and appropriate patient selection for optimal outcomes.
Table of content
- 1. Scientific mechanisms drive therapeutic effects
- 2. Clinical evidence shows promise with important limitations
- 3. Practical implementation requires careful device selection and protocols
- 4. FDA regulation provides framework for medical claims
- 5. Current evidence reveals both promise and critical gaps
- 6. Recommendations balance evidence with practical considerations
- 7. Conclusion
- 8. References
Scientific mechanisms drive therapeutic effects
Red light therapy operates through photobiomodulation (PBM), where specific wavelengths of light (630-850nm) interact with cellular chromophores to produce biological effects. The primary mechanism involves cytochrome c oxidase (CCO), the terminal enzyme in mitochondrial electron transport, which serves as the key photoacceptor for red and near-infrared wavelengths.
When light photons interact with CCO, they dissociate inhibitory nitric oxide from the enzyme's active sites, immediately restoring cellular respiration and increasing ATP production by 2-3 fold. This enhanced mitochondrial function triggers downstream cellular effects including reduced inflammation, enhanced tissue repair, and improved cellular metabolism. The therapy demonstrates biphasic dose-response relationships following the Arndt-Schulz law, where optimal therapeutic doses (1-10 J/cm²) produce beneficial effects, while excessive doses can cause inhibitory responses.
Red light (630nm+) primarily affects surface tissues with 2-5mm penetration depth, making it optimal for skin applications, wound healing, and collagen stimulation. Near-infrared wavelengths (810-850nm) achieve superior tissue penetration of 5-15mm, enabling treatment of deeper structures like muscle, joints, and neural tissue. These wavelength-specific effects explain why combined red/NIR protocols often demonstrate enhanced therapeutic outcomes compared to single-wavelength treatments.
Beyond mitochondrial effects, emerging research identifies additional mechanisms including ion channel activation, mechanotransduction pathways, and direct extracellular matrix interactions that contribute to therapeutic responses.
Clinical evidence shows promise with important limitations
The clinical evidence base for red light therapy includes over 4,000 studies indexed in PubMed, with numerous systematic reviews and meta-analyses published between 2020-2025. However, evidence quality varies significantly across different medical applications, with methodological limitations affecting interpretation and clinical translation.
Androgenic alopecia represents the strongest evidence base, with multiple systematic reviews demonstrating consistent positive results for hair regrowth in both men and women. FDA-cleared devices show efficacy comparable to minoxidil, with best results when combined with pharmacological treatments using 660-850nm wavelengths.
For pain management, moderate evidence supports short-term relief in conditions like knee osteoarthritis (SMD = 0.96, 95% CI 0.31-1.61 vs. sham), rheumatoid arthritis morning stiffness, and plantar fasciitis. However, evidence for chronic pain conditions remains limited, with insufficient data on long-term effectiveness.
Wound healing shows promising results with meta-analyses demonstrating significant improvements in burn wound contraction and general wound healing parameters. Effective doses range from 0.1-10 J/cm² across wavelengths of 405-1,000nm, though protocol standardization remains a challenge.
Oral mucositis prevention in cancer patients represents one of the most established applications, with "silver level" evidence recognized by international guidelines (MASCC/ISOO) for reducing treatment-related side effects.
Areas with limited or conflicting evidence include acne treatment (meta-analysis showing no significant difference from conventional therapies), cognitive enhancement (small studies requiring validation), and athletic performance (mixed results with high protocol variability).
Recent breakthrough studies have identified childhood myopia control as an emerging strong application, with large-scale RCTs showing 53.3% of high myopia patients experiencing substantial improvement with repeated low-level red light therapy.
Practical implementation requires careful device selection and protocols
Successful red light therapy implementation depends on appropriate device selection, standardized protocols, and adherence to safety guidelines. The market offers devices ranging from $25 consumer LED masks to $25,000+ professional medical systems, each with distinct capabilities and applications.
Consumer devices typically provide 10-100 mW/cm² power density with basic wavelength options (usually 630nm and 830nm), suitable for home use with longer treatment times. Professional medical-grade systems offer 50-200 mW/cm² power density, multi-wavelength capabilities, and precise dosimetry control, enabling shorter treatment sessions with enhanced efficacy.
Critical device specifications include wavelength accuracy (±10nm tolerance), appropriate power density for intended applications, medical-grade LED quality, and safety certifications. Multi-wavelength systems combining 630nm, 660nm, 810nm, 830nm, and 850nm provide optimal flexibility for different treatment protocols.
Standard treatment protocols vary by condition: skin applications typically use 6-12 inches distance for 10-20 minutes, 3-5 times weekly at 1-10 J/cm² dose. Deep tissue applications require 2-6 inches distance for 15-30 minutes, often daily for acute conditions, using 10-50 J/cm² doses with 810-850nm wavelengths.
The therapy maintains an excellent safety profile with minimal adverse effects when used properly. Absolute contraindications include direct eye exposure (requiring protective eyewear), active cancer sites without oncologist approval, and pregnancy for abdominal treatments. Most devices operate non-thermally below 104°F skin temperature, eliminating burn risks associated with higher-powered laser systems.
FDA regulation provides framework for medical claims
Red light therapy devices fall under FDA medical device regulations, with most receiving Class I or Class II designation requiring 510(k) clearance for medical claims. FDA-cleared applications include pain relief, temporary muscle tension reduction, increased local blood circulation, and specific dermatological conditions.
However, regulatory compliance remains challenging as many manufacturers incorrectly claim "FDA approval" rather than the accurate "FDA clearance" designation. The FDA has issued warning letters to companies making unsupported medical claims, particularly for weight loss, cellulite reduction, and mental health applications.
Insurance coverage remains limited, with most plans excluding red light therapy as "investigational" or "cosmetic." Professional treatments typically cost $25-$300 per session, with complete protocols ranging $1,000-$4,500. Home devices offer cost-effective alternatives for chronic conditions requiring ongoing treatment.
Current evidence reveals both promise and critical gaps
Red light therapy stands at a critical juncture where commercial success has outpaced rigorous clinical validation. The field demonstrates legitimate therapeutic potential supported by solid mechanistic understanding, but significant research gaps limit broader medical adoption.
Methodological heterogeneity represents the greatest challenge, with wide variations in wavelengths (630-1,000nm), power densities (0.1-1.5 W/cm²), treatment protocols, and outcome measures across studies. This inconsistency makes clinical translation difficult and prevents establishment of standardized treatment guidelines.
Evidence quality limitations include small sample sizes (many studies <50 participants), short-term follow-up periods, blinding difficulties, and potential publication bias favoring positive results. While the volume of research is substantial, the quality often falls short of standards required for mainstream medical adoption.
Recent developments show promise for breakthrough applications including childhood myopia control and age-related macular degeneration, with properly designed large-scale trials demonstrating clinically significant outcomes. These successes provide templates for future research methodology.
Recommendations balance evidence with practical considerations
For healthcare providers, red light therapy represents a low-risk adjuvant treatment option for specific conditions with established evidence. Hair loss, acute pain management, and wound healing show sufficient evidence to support clinical use when proper FDA-cleared devices and evidence-based protocols are employed.
Providers should select Class II medical devices with multi-wavelength capabilities and power densities exceeding 50 mW/cm² at 6 inches. Standardized treatment protocols based on FDA-cleared indications, proper staff training, and detailed outcome documentation are essential for professional implementation.
Individual users should focus on FDA-cleared devices for intended applications, with medical consultation before starting treatment. Home devices in the 25-100 mW/cm² range with built-in safety features provide reasonable efficacy for appropriate conditions when used consistently.
Conditions requiring more research include acne treatment, cognitive enhancement, and long-term pain management, where evidence remains insufficient to support routine clinical use. Patients should be counseled on realistic expectations and evidence limitations.
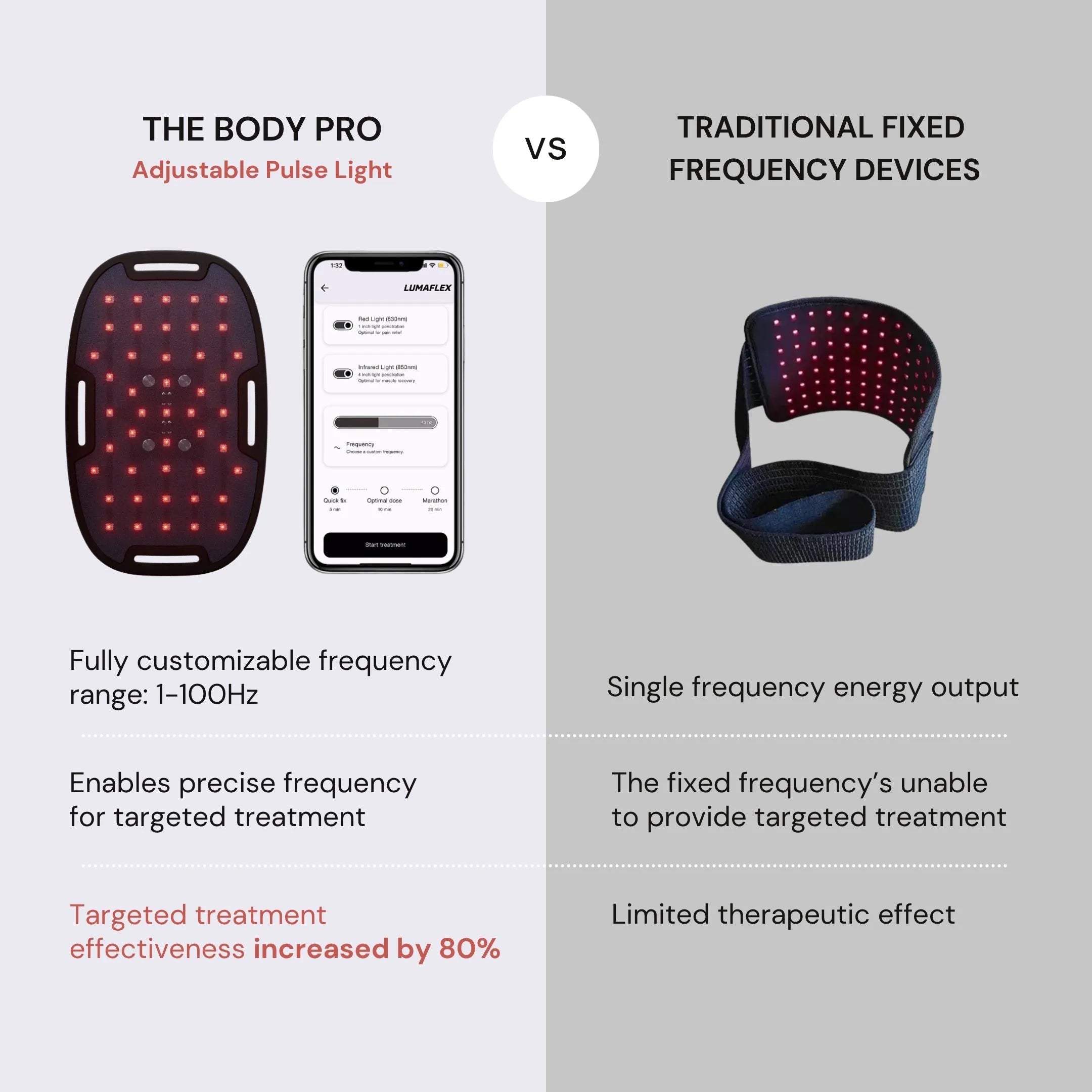
The Lumaflex Body Pro is an FDA cleared RLT device using red light wavelength 630nm + infrared wavelength 850nm to relieve pain effectively. It is a comprehensive, reliable, and effective solution with 10 international quality certifications and 9 design awards.
Conclusion
Red light therapy has emerged as a legitimate therapeutic modality with moderate-to-strong evidence for specific applications, particularly hair loss, pain management, and wound healing. The therapy's excellent safety profile, non-invasive nature, and FDA regulatory framework support its integration into evidence-based medical practice for appropriate conditions.
However, the field requires standardization of treatment protocols, larger adequately powered clinical trials, and better regulatory oversight of consumer devices to fulfill its therapeutic potential. Success depends on balancing commercial enthusiasm with scientific rigor, focusing research efforts on applications with the strongest mechanistic rationale and preliminary evidence.
For healthcare providers and patients, red light therapy offers a valuable adjuvant treatment option when properly implemented with appropriate device selection, evidence-based protocols, and realistic expectations about therapeutic outcomes.
References
- Mosca RC, Ong AA, Albasha O, Bass K, Arany P. Photobiomodulation Therapy for Wound Care: A Potent, Noninvasive, Photoceutical Approach. Adv Skin Wound Care. 2019 Apr;32(4):157-167. doi: 10.1097/01.ASW.0000553600.97572.d2. PMID: 30889017.
- Kuffler DP. Photobiomodulation in promoting wound healing: a review. Regen Med. 2016 Jan;11(1):107-22. doi: 10.2217/rme.15.82. Epub 2015 Dec 18. PMID: 26681143.
- Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337-361. doi: 10.3934/biophy.2017.3.337. Epub 2017 May 19. PMID: 28748217; PMCID: PMC5523874.
- Márcia Cristina Prado Felician, Renata Belotto, João Paulo Tardivo, Mauricio S. Baptista, Waleska Kerllen Martins, Photobiomodulation: Cellular, molecular, and clinical aspects, Journal of Photochemistry and Photobiology, Volume 17, 2023, 100197, ISSN 2666-4690, https://doi.org/10.1016/j.jpap.2023.100197.
- de Freitas LF, Hamblin MR. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J Sel Top Quantum Electron. 2016 May-Jun;22(3):7000417. doi: 10.1109/JSTQE.2016.2561201. PMID: 28070154; PMCID: PMC5215870.
- Hamblin MR. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol. 2018 Mar;94(2):199-212. doi: 10.1111/php.12864. Epub 2018 Jan 19. PMID: 29164625; PMCID: PMC5844808.
- Chichan H, Aldujaly IH, Michalakis K, Kanal L. Photobiomodulation in ocular therapy: current status and future perspectives. Int J Ophthalmol. 2025 Feb 18;18(2):351-357. doi: 10.18240/ijo.2025.02.20. PMID: 39967973; PMCID: PMC11754031.
- Cotler HB, Chow RT, Hamblin MR, Carroll J. The Use of Low Level Laser Therapy (LLLT) For Musculoskeletal Pain. MOJ Orthop Rheumatol. 2015;2(5):00068. doi: 10.15406/mojor.2015.02.00068. Epub 2015 Jun 9. PMID: 26858986; PMCID: PMC4743666.
- Glass GE. Photobiomodulation: The Clinical Applications of Low-Level Light Therapy. Aesthet Surg J. 2021 May 18;41(6):723-738. doi: 10.1093/asj/sjab025. Erratum in: Aesthet Surg J. 2022 Apr 12;42(5):566. doi: 10.1093/asj/sjab396. PMID: 33471046.
- Liebert A, Capon W, Pang V, Vila D, Bicknell B, McLachlan C, Kiat H. Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine. Biomedicines. 2023 Jan 17;11(2):237. doi: 10.3390/biomedicines11020237. PMID: 36830774; PMCID: PMC9953702.
- Yang M, Yang Z, Wang P, Sun Z. Current application and future directions of photobiomodulation in central nervous diseases. Neural Regen Res. 2021 Jun;16(6):1177-1185. doi: 10.4103/1673-5374.300486. PMID: 33269767; PMCID: PMC8224127.
- Hamblin MR. Photobiomodulation for the management of alopecia: mechanisms of action, patient selection and perspectives. Clin Cosmet Investig Dermatol. 2019 Sep 6;12:669-678. doi: 10.2147/CCID.S184979. PMID: 31686888; PMCID: PMC6737896.
- González-Muñoz A, Cuevas-Cervera M, Pérez-Montilla JJ, Aguilar-Núñez D, Hamed-Hamed D, Aguilar-García M, Pruimboom L, Navarro-Ledesma S. Efficacy of Photobiomodulation Therapy in the Treatment of Pain and Inflammation: A Literature Review. Healthcare (Basel). 2023 Mar 24;11(7):938. doi: 10.3390/healthcare11070938. PMID: 37046865; PMCID: PMC10094541.
- Deana NF, Alves N, Zaror C, Del Sol M, Bagnato VS. Photobiomodulation Therapy in Burn Wound Healing: Systematic Review and Meta-Analysis of Preclinical Studies. Photobiomodul Photomed Laser Surg. 2021 Jul;39(7):439-452. doi: 10.1089/photob.2020.4972. PMID: 34264767.
- Zhang G, Yi L, Wang C, Yang P, Zhang J, Wang J, Lu C, Zhang X, Liu Y. Photobiomodulation promotes angiogenesis in wound healing through stimulating the nuclear translocation of VEGFR2 and STAT3. J Photochem Photobiol B. 2022 Dec;237:112573. doi: 10.1016/j.jphotobiol.2022.112573. Epub 2022 Sep 22. PMID: 36403534.
- Robijns J, Nair RG, Lodewijckx J, Arany P, Barasch A, Bjordal JM, Bossi P, Chilles A, Corby PM, Epstein JB, Elad S, Fekrazad R, Fregnani ER, Genot MT, Ibarra AMC, Hamblin MR, Heiskanen V, Hu K, Klastersky J, Lalla R, Latifian S, Maiya A, Mebis J, Migliorati CA, Milstein DMJ, Murphy B, Raber-Durlacher JE, Roseboom HJ, Sonis S, Treister N, Zadik Y, Bensadoun RJ. Photobiomodulation therapy in management of cancer therapy-induced side effects: WALT position paper 2022. Front Oncol. 2022 Aug 30;12:927685. doi: 10.3389/fonc.2022.927685. PMID: 36110957; PMCID: PMC9468822.
- Ebrahimi P, Hadilou M, Naserneysari F, Dolatabadi A, Tarzemany R, Vahed N, Nikniaz L, Fekrazad R, Gholami L. Effect of photobiomodulation in secondary intention gingival wound healing-a systematic review and meta-analysis. BMC Oral Health. 2021 May 13;21(1):258. doi: 10.1186/s12903-021-01611-2. PMID: 33985492; PMCID: PMC8120828.
- Wu Y, Deng Y, Huang P. Application of red light therapy for moderate-to-severe acne vulgaris: A systematic review and meta-analysis. J Cosmet Dermatol. 2021 Nov;20(11):3498-3508. doi: 10.1111/jocd.14369. Epub 2021 Aug 7. PMID: 34363730.
- Ngoc LTN, Moon JY, Lee YC. Utilization of light-emitting diodes for skin therapy: Systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2023 Jul;39(4):303-317. doi: 10.1111/phpp.12841. Epub 2022 Nov 9. PMID: 36310510.
- Tang J, Liao Y, Yan N, Dereje SB, Wang J, Luo Y, Wang Y, Zhou W, Wang X, Wang W. Efficacy of Repeated Low-Level Red-Light Therapy for Slowing the Progression of Childhood Myopia: A Systematic Review and Meta-analysis. Am J Ophthalmol. 2023 Aug;252:153-163. doi: 10.1016/j.ajo.2023.03.036. Epub 2023 Apr 7. PMID: 37030495.
- Salzano AD, Khanal S, Cheung NL, Weise KK, Jenewein EC, Horn DM, Mutti DO, Gawne TJ. Repeated Low-level Red-light Therapy: The Next Wave in Myopia Management? Optom Vis Sci. 2023 Dec 1;100(12):812-822. doi: 10.1097/OPX.0000000000002083. Epub 2023 Oct 25. PMID: 37890098.
- Glass GE. Photobiomodulation: A Systematic Review of the Oncologic Safety of Low-Level Light Therapy for Aesthetic Skin Rejuvenation. Aesthet Surg J. 2023 Apr 10;43(5):NP357-NP371. doi: 10.1093/asj/sjad018. PMID: 36722207; PMCID: PMC10309024.
- Photobiomodulation (PBM) Devices - Premarket Notification [510(k)] Submissions - FDA
- Wunsch A, Matuschka K. A controlled trial to determine the efficacy of red and near-infrared light treatment in patient satisfaction, reduction of fine lines, wrinkles, skin roughness, and intradermal collagen density increase. Photomed Laser Surg. 2014 Feb;32(2):93-100. doi: 10.1089/pho.2013.3616. Epub 2013 Nov 28. PMID: 24286286; PMCID: PMC3926176.
- Rassi, T.N., Barbosa, L.M., Pereira, S. et al. Photobiomodulation efficacy in age-related macular degeneration: a systematic review and meta-analysis of randomized clinical trials. Int J Retin Vitr10, 54 (2024). https://doi.org/10.1186/s40942-024-00569-x
- Hernández-Bule, M.L.; Naharro-Rodríguez, J.; Bacci, S.; Fernández-Guarino, M. Unlocking the Power of Light on the Skin: A Comprehensive Review on Photobiomodulation. Int. J. Mol. Sci.2024, 25, 4483. https://doi.org/10.3390/ijms25084483